To sign up for our daily email newsletter, CLICK HERE
Morphologic Features and Classification of the Virus
Viruses have been known to exist since the beginning of life. However, the specific origin of viruses is yet to determine. The size of viruses may range from 1 µm (Ebola virus) to 20 nm (canine micro virus). The genetic material of viruses may be RNA or DNA existing as a double- or single-stranded genome. Viruses may exist in the form of spherical or rod shapes. For coronavirus, the size of this virus lies within the range of 1 µm-20 nm. The shape and other morphological properties of coronavirus are similar to the virus that causes severe acute respiratory syndrome (SARS).
Coronaviruses belong to the vertebrate virus family Coronaviridae, with the genetic material composed of single-stranded RNA. This family comprises a total of seven different coronaviruses. Bats are the natural hosts for five viruses, while wild rodents are natural hosts for only two coronaviruses. The Coronaviridae family viruses are responsible for causing SARS and the Middle East respiratory syndrome (MERS). The coronavirus is classified under the order Nidovirales. Vertebrates serve as hosts for the coronavirus, which uses its genetic machinery and the cellular system of the host to synthesize proteins.
Following the Wuhan outbreak, scientists discovered that approximately 7 coronaviruses could infect humans. Out of these, 4 coronaviruses do not cause symptomatic disease in healthy individuals. Symptoms associated with the infection of these viruses are observed in the background of inadequate host immunity. On the contrary, the viruses responsible for causing SARS and MERS may lead to serious clinical presentation. The mortality rates of SARS and MERS are approximately 9.6% and 35%, respectively.
MERS-CoV, SARS-CoV, and 2019-nCoV are the three viruses of the Coronaviridae family that pose a significant threat to the health of humans, with the origin attributed to bats. Under normal circumstances, the virus cannot transmit directly from bats to humans for two reasons – bats live farther away from human-inhabited areas and require other organisms for the virus to become capable of infecting humans. However, humans may acquire the virus directly by consuming the meat of infected hosts.
The coronavirus has several peculiar features when observed under the electron microscope. Western crown of the coronavirus attributes to the name of coronaviruses. Coronavirus envelope is characterized by crown edges appearing as spikes or protrusions that are made of glycoproteins. These glycoproteins are synthesized by the viral RNA’s Spike (S) gene. The increased protein content of the viral envelope predisposes this structure to degradation by alcohol since 75% alcohol denatures proteins with subsequent destruction of the virus. To maintain genomic stability, nucleoproteins bind to the viral RNA. Finally, 1/3rd of the viral RNA contains genes that encode for structural proteins, whereas the remaining 2/3rd of the viral RNA encodes for replication-related enzymes.
Analysis of the viral genome of the 2019-nCoV revealed a similarity of 96.2% with bat coronavirus and 79.5% with SARS virus. The 2019-nCoV virus enters the human bronchial and alveolar cells by interaction of spinosin S1 protein with cell surface ACE2, followed by spinosin S2 subunit-mediated endocytosis into the cell. As opposed to DNA double helix, single-stranded RNA mutates rapidly, causing the virus to evolve at an extremely high rate, thus making the management and prevention of viral infections a challenging task.
How Does Coronavirus Infect the Cell?
- The viral spinosin protein interacts with the surface proteins of the host cell, leading to endocytosis of the virus into the host cell.
- Lysosome in the host cell mediates the lysis of the viral envelope.
- Viral RNA uses cellular translation machinery to transcribe specific genes into replication-related enzymes.
- The viral RNA also replicates itself simultaneously.
- The viral encoded enzymes modulate the translation of viral proteins using the cellular endoplasmic reticulum.
- The viral parts are assembled to form a complete virus.
- The endoplasmic reticulum, containing the virus, fuses with the cell membrane to release the virus.
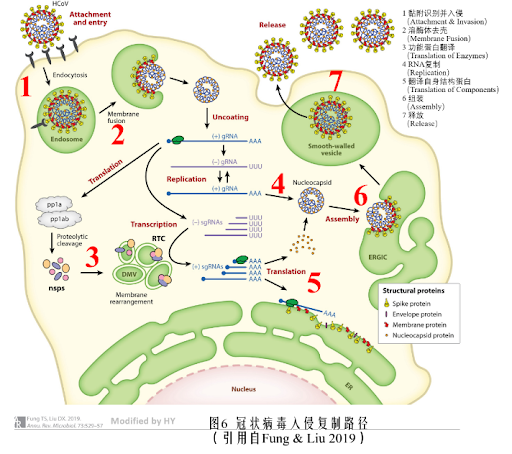
The drugs formulated to prevent and contain the infection may intervene in the abovementioned steps and control the disease.
Antiviral Therapy
The antiviral approaches to control and prevent coronavirus infection are broadly classified into chemotherapy, vaccine therapy, and serum/antibody therapy.
1. Serum/Antibody Therapy
Cured patients are believed to synthesize their antibodies against the 2019-nCoV found in the blood. The direct transfer of antibody-containing serum of these patients to infected individuals may lead to a severe immune reaction, including rejection in the antibody recipients, making this therapeutic technique an unrealistic option for the given large pool of patients.
2. Vaccine Therapy
Vaccines are an effective therapeutic technique for double-helix DNA viruses owing to genomic stability in these viruses. However, single-stranded RNA viruses are known to mutate at an extremely high frequency, making their control and prevention a difficult process. The antigenic cluster administered by a particular vaccine may not elicit immunity for the subsequent mutations and resultant antigenic determinants, making the memory cells and immune response useless.
3. Drug Therapy
The most time-efficient and promising treatment modality for coronavirus infection is drug therapy. Gilead has developed two drugs that have been approved for clinical trials. An adenosine analog, raltegravir, terminates the RNA replication and limits viral proliferation. The second drug, chloroquine, blocks viral entry into the host cells. At the Wuhan Institute of Virology, an antiviral peptide called EK1 has been developed to block the entry of five coronaviruses into the host cell. While developing drugs against coronavirus, scientists must ensure that the pharmacologic formulation does not target the normal protein structures of healthy host cells.
The host proteins involved in the pathogenesis of coronavirus and can be a potential target for drugs include ACE2, GSK3, hnRNPA1, NL-GE, ER molecular chaperones, and TMPRSS11D. Since the invasive mechanisms of 2019-nCoV are yet to be identified, the formulation of drugs specific to 2019-nCoV has not been accomplished.
The exchange of viral genomes between different hosts causes the virus to adapt more effectively to newer hosts. This results in highly intensive infectivity and unpredictable outcomes of viral infection in the newer hosts.
Lessons to Learn
To reduce and prevent the invasion of non-human viruses into humans, one shall refrain from both direct and indirect contact with wild animals. The healthcare and administrative bodies shall invest more in pharmaceutical research to equip the nation with sufficient weapons and tools to fight off and prevent the virus and other disease outbreaks. In addition, the use of deep learning and artificial intelligence tools in conjunction with the available viral genome and mutation data can help scientists predict the host migration, pathogenesis and devise drugs to treat the viral infection, minimizing the anticipated damage.
Endnote
The recent wave of 2019-nCoV has caused severe harm to mankind. With the discovery of the first 2019-nCoV case, researchers, scientists, healthcare professionals, bioengineers, and other professionals have worked hard to derive effective management, treatment, and prevention strategies. Accurate animal models facilitate research, vaccine development, gene therapy, and drug design as well as aid the healthcare personnel in identifying the disease’s pathogenesis and host immune response. Cyagen is an animal modeling service provider that has taken the responsibility to develop accurate animal models for research related to coronavirus outbreaks.
The coronavirus binds to cell surface receptors, including ACE2, DPP4, and APN, and invades the host cells. Research and drug development associated with these three cell surface proteins are highly relevant to the control and prevention of the current outbreak.
References
- To Sing Fung and Ding Xiang Liu. Human Coronavirus: Host-Pathogen Interaction. Annual Review of Microbiology. vol. 73, (2019)
- Zhou, P. et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature https://doi.org/10.1038/s41586-020-2012-7, ( 2020).
- Wu, F. et al. A new coronavirus associated with human respiratory disease in China. Nature https://doi. org/10.1038/s41586-020-2008-3, (2020).
- Jie Cui et al. Origin and evolution of pathogenic coronaviruses. nature reviews. (2019)
- Shibo Jiang et al. An emerging coronavirus causing pneumonia outbreak in Wuhan, China: calling for developing therapeutic and prophylactic Emerging microbes&infections. (2020)
- Chan et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster, The Lancet, (2020)